UNDP is taking a lead globally to support countries in the most challenging operating environments to procure health products and strengthen national supply chain systems, in close collaboration with other partners. UNDP’s expertise built through more than 15 years of implementing procurement and supply chain (PSM) support services under donor grants is being used to support ongoing reforms of national procurement strategies and operational policies, and to optimize national supply chain systems for health products.
Overview
UNDP supports countries in some of the most challenging operating environments to procure health products and strengthen national supply chain systems, working in close collaboration with other partners. UNDP’s expertise, built through more than 15 years of implementing procurement and supply chain management (PSM) support services under donor grants, is being used to support ongoing reforms of national procurement strategies and operational policies, and to optimize national supply chain systems for health products.
UNDP builds on its support to governments’ procurement of health products as an entry point to improve and build resilience in PSM systems in countries. While supporting the timely procurement of quality-assured medicines and other health products, UNDP works with governments, in coordination with the World Health Organization and other partners, to help enhance national capacities and systems for the adequate PSM through transparent and accountable mechanisms.
It helps countries to achieve value-for-money in procurement and supply chain activities, it guarantees the quality of the products and services provided, and it sustainably improves the performance of national procurement and supply chain systems using both donors and domestic funds.
UNDP’s approach
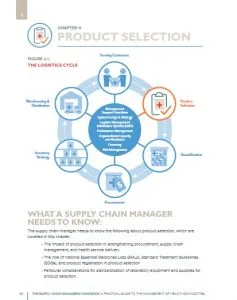
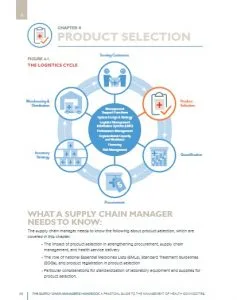
UNDP’s approach to procurement and supply chain management capacity development is built on a participatory and inclusive process. It seeks to address capacity gaps at all levels of the supply chain, including selection, quantification, procurement, storage, distribution, logistics management and the use of health products, with a cross-cutting emphasis on meeting quality assurance standards, fostering enabling legal and regulatory environments, and promoting environmental sustainability in PSM.
UNDP supports situation analyses and national supply chain maturity assessments to enable the development of national PSM strategies and improvement plans. The approach engages the contributions of technical partners to define the national priorities and develop integrated solutions for national supply chains.
UNDP strongly encourages the establishment of national coordination platforms under the leadership of national authorities (e.g. the ministry of health or supply chain entities) to steer the implementation of supply chain transformation projects and monitor progress and performance. All national and international stakeholders should be part of the coordination mechanism. As a member of the coordination platform, UNDP will support the implementation of prioritized activities in close collaboration with national counterparts to strengthen national leadership and management. It likewise provides project management support to ministry of health counterparts to lead the capacity development projects, measure progress, identify risks and propose mitigation measures.
Potential activities through which UNDP might assist national and subnational actors across the supply chain include technical assistance to:
- define the relevant specifications and appropriate quality standard requirements for selected products
- quantify needs based on reliable programmatic and logistic information
- carry out sourcing and supply planning and conduct procurement processes
- store and distribute health products while monitoring their quality
- establish logistic information systems with analytic capabilities for stock visibility and security and logistic data reliability, for informed supply planning decisions
- design solutions for “last-mile” delivery up to the most hard-to-reach populations
- strengthen policies and regulatory frameworks, including on intellectual property, to remove potential barriers to equitable access to affordable medicines and other health products.
UNDP has also been able to leverage its global procurement architecture and expertise to support health procurement efforts within national COVID-19 response plans, in coordination with other agencies. It has helped countries to procure quality-assured products, including personal protective equipment, medical equipment, test kits and ventilators, including through funding from the Global Fund to Fight AIDS, Tuberculosis and Malaria (The Global Fund) COVID-19 Response Mechanism.
Cross-cutting tools, processes and frameworks for strengthening procurement and supply chain management systems
This section provides guidance and tools to build capacities at all stages of the procurement and supply chain management (PSM) cycle. Some examples of tools, approaches and resources in cross-cutting areas of PSM strengthening, such as human resources, innovation and sustainability, can be found below.
Human resources for procurement and supply chain management
UNDP recognizes that a knowledgeable, skilled, motivated workforce is crucial for implementing procurement and supply chain management (PSM) strategies and plans. Human resources for health strategies are a fundamental part of developing resilient PSM systems and should encompass sustainable solutions for the education, recruitment and retention of human resources for PSM.
In several countries, UNDP has strengthened human resources through a scaling-up of on-the-job and formal training sessions, including through professional certification programmes for health workers in PSM-related areas, such as accreditation from the internationally recognized Chartered Institute of Purchasing and Supply (CIPS) and supply chain leadership programmes with partner organizations.
UNDP has also developed online introductory training courses on PSM, accessible in four United Nations languages. The training sessions are intended to support the capacity development of health system personnel and partners by increasing understanding of the importance of PSM to ensure the uninterrupted supply of life-saving medicines and other health products.
UNDP can additionally support countries to mobilize expertise and technical assistance to reinforce human resources through international consultants where relevant. To strengthen its capacity to effectively support partners worldwide, UNDP has established a pre-approved health PSM roster of experts and senior experts, who are ready to provide consultancy services for sustainable development in PSM, specifically pertaining to the health sector. The health PSM roster contains qualified individual consultants based on competencies and value-for-money principles that may be called upon to be contracted and deployed to provide specific technical advice and short-term consultancies for periods of time, normally not exceeding 12 months.
Innovations in procurement and supply chain management
UNDP seeks out and uses the latest innovation and technology for health, which is central to supporting resilient and sustainable systems for health. UNDP is involved in piloting and implementing several innovations in the supply chain. These include:
- Upstream innovation for sustainable procurement, such as engaging manufacturers through its long-term agreements, optimizes medicine packaging to reduce waste and freight costs.
- Supporting mobile phone-based or other electronic logistic management information systems (eLMIS) allows the tracking of inventory, the consumption of data and the monitoring of cold chain temperature for vaccines at the most peripheral storage level. Read more
- The Solar for Health initiative leverages solar energy to ensure the sustainable provision of electricity to pharmaceutical warehouses and health facilities. This guarantees not only the functioning of cold chain equipment but also the storage of health products at controlled temperature and electricity for computer-based warehouse management systems and LMIS, among other things.
- Promoting the adoption of the Global Data Standards (GS1) improves the traceability of health products along national supply chains, through joint efforts with other agencies of the Interagency Supply Chain Group (ISG). This also improves supply chain security and reduces the risk of falsified health products being introduced into the national supply chain.
- Global partnerships to facilitate coordination and learning on the latest technologies for procurement and supply chain management (PSM). UNDP is participating with other agencies in global coordination mechanisms, such as the Interagency Supply Chain Group (ISG) and Sustainable Procurement in the Health Sector (SPHS), to introduce innovations and the use of technologies in PSM.
Additional initiatives can be found in the ‘Innovation and digital technologies’ section, include the application of Smart Facilities to strengthen the sustainability of physical infrastructure in supply chains.
A procurement architecture to create value for money
UNDP’s principles for guiding health procurement are:
- provide the best value for money;
- embody fairness, integrity, and transparency;
- engage in effective international competition.
UNDP has developed and continues to manage a procurement architecture designed to facilitate the timely supply of affordable quality assured pharmaceutical and other health products to meet the needs of national programmes using donor grants and domestic funds for health procurement supported by UNDP through a value-for-money service proposition. The UNDP health procurement architecture comprises several partnerships and sourcing agreements with other United Nations agencies, manufacturers and other commercial entities to provide the most cost-efficient procurement system for each health product category.
As a result, UNDP health procurement has achieved competitive prices for health products. These savings are reinvested to support increased health service coverage or strengthen national supply chain systems. Similarly, UNDP’s capacity-building for in-country procurement processes and operations aims to achieve efficiencies to be reinvested to strengthen other areas of the procurement and supply chain management cycle.
UNDP is also working to reduce monetary and environmental costs related to transport, health care waste, and packaging. This likewise promotes value for money in health procurement for recipient countries. Increasingly, this type of approach is meant to be transferred and applied in country-led procurement processes supported by UNDP.
The Global Fund investments to strengthen global and in-country procurement and supply chain management systems
UNDP works closely with the Global Fund in a number of countries to implement capacity development interventions for procurement and supply chain management (PSM). Improved access to essential medicines and health products is critical to fighting HIV, tuberculosis and malaria and is recognized as a key building block of a strong system for health. Weak procurement and ineffective supply chains reduce the overall health system’s ability to respond to the health-care needs of the population. As part of its strategy, “The Global Fund Strategy 2023–2028: Fighting Pandemics and Building a Healthier and More Equitable World”, the Global Fund prioritizes investments in building Resilient and Sustainable Systems for Health including capacity development for PSM systems